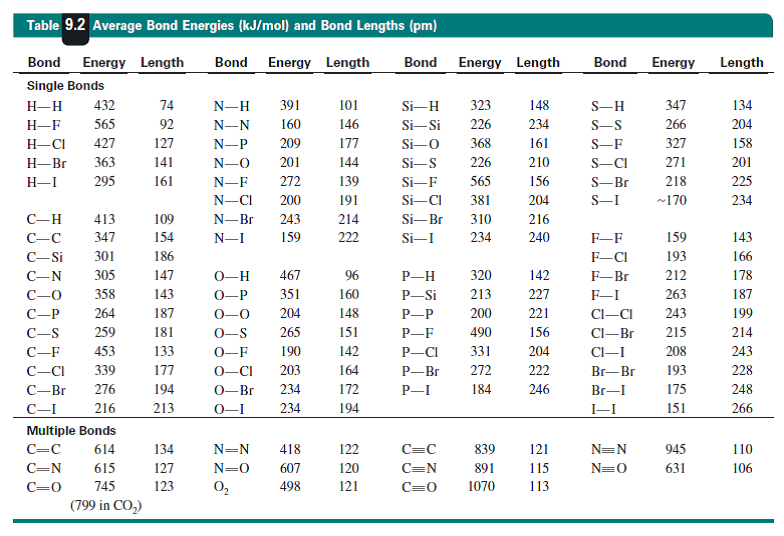
Table 11 from Theoretical studies of transition-metal hydrides. 1. Bond energies for MH+ with M = Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, and Zn | Semantic Scholar

Universal Quantification of Chemical Bond Strength and Its Application to Low Dimensional Materials | IntechOpen

SOLVED: Calculate the bond energy for the formation of boron trifluoride: L Average Bond Enthalpies (kJ/mol) Single Bonds: C-H 413 391 348 163 293 201 358 272 485 200 328 243 463
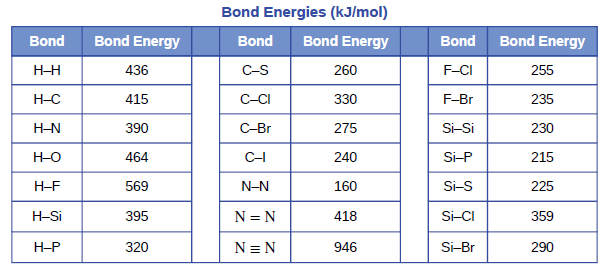
Using the bond energies in Table 7.2, determine the approximate enthalpy change for each of the following reactions: (a) Cl 2 ( g ) + 3 F 2 ( g ) →
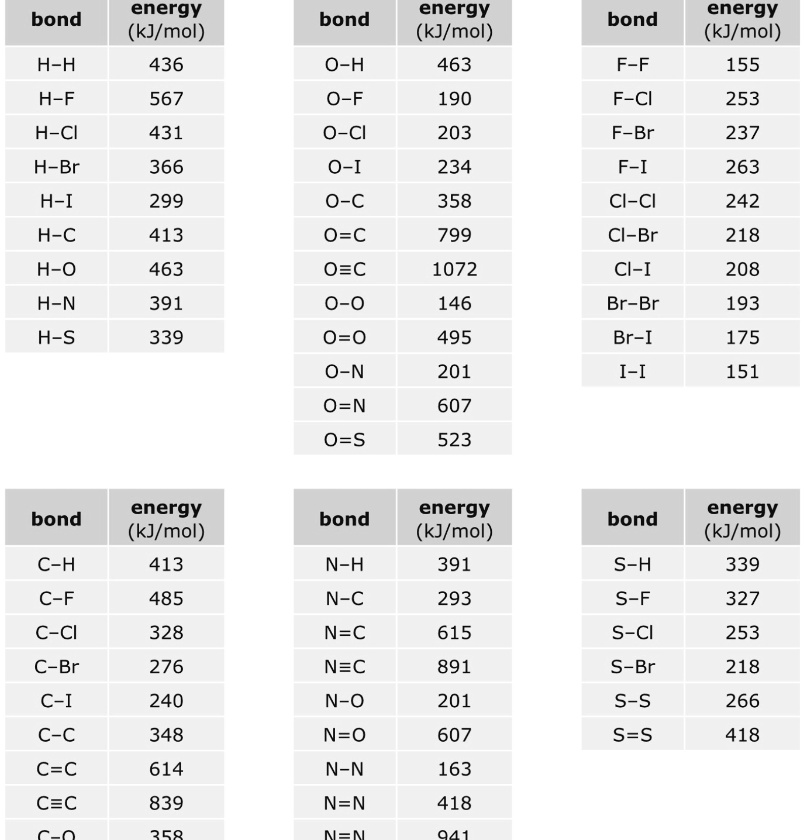
SOLVED: Bond Energy (kJ/mol): 436, 567 Bond Energy (kJ/mol): 463 Bond Energy (kJ/mol): 155, 253, 237 H-H O-H F-F H-F O-F 190 F-Cl H-Cl 431 O-Cl 203 F-Br H-Br 366, 299 O-I
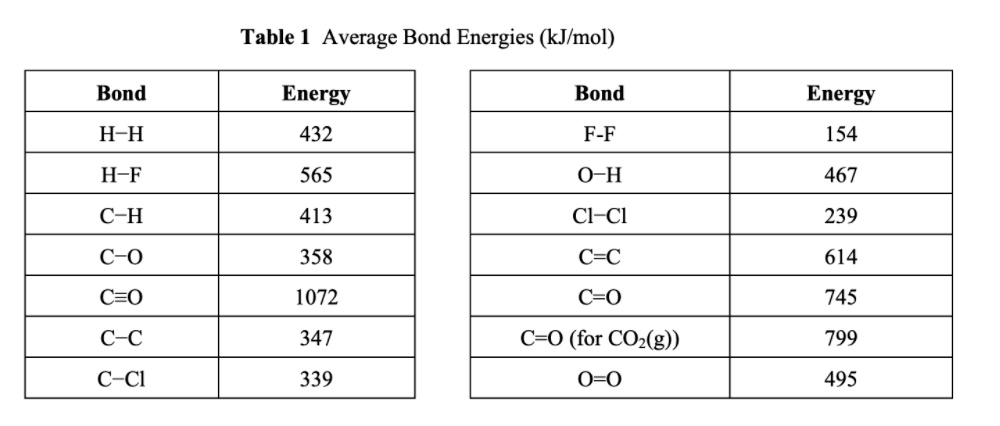





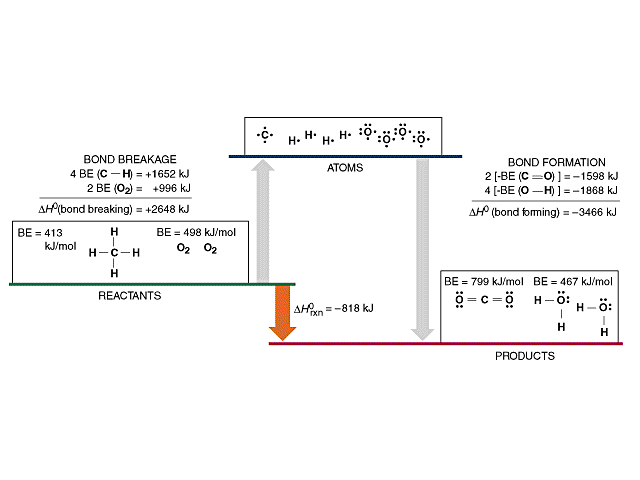

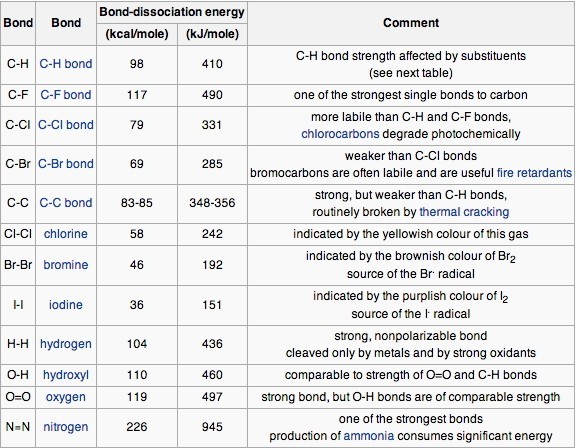
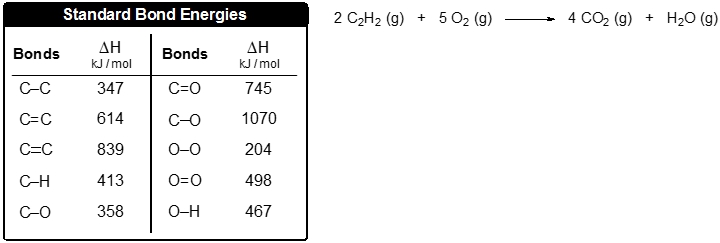
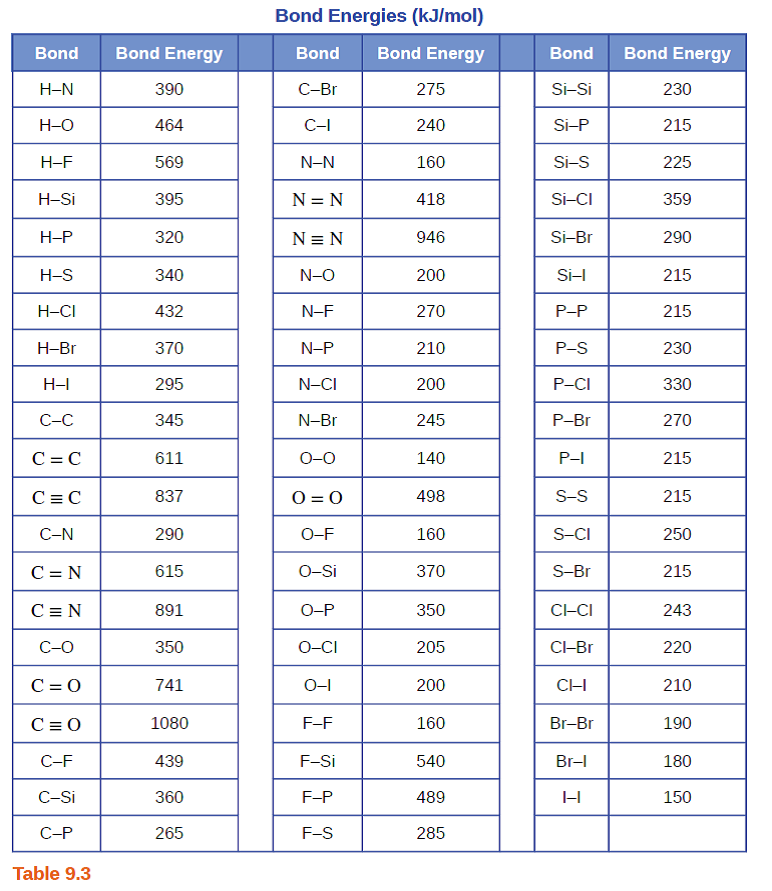

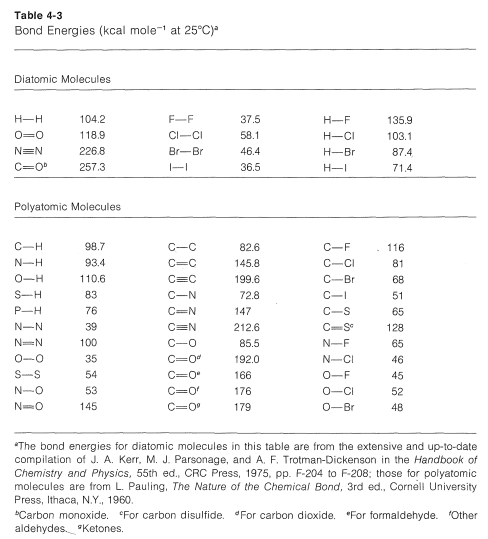

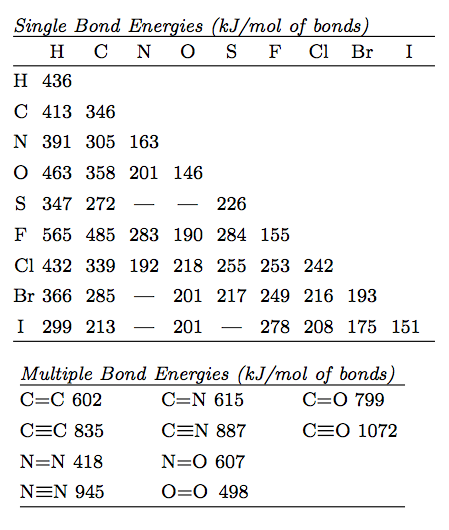

![PDF] Bond dissociation energies | Semantic Scholar PDF] Bond dissociation energies | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/ce7c065d005590afbab6bb30de466e75e50d4f86/7-Table3-1.png)